Dalton's Law of Partial Pressure
Daltons law of partial pressures has a lot of applicationsIt is one of the most conventional methods to measure the pressure exerted by two gases and calculate the partial and total. If the water levels within and.
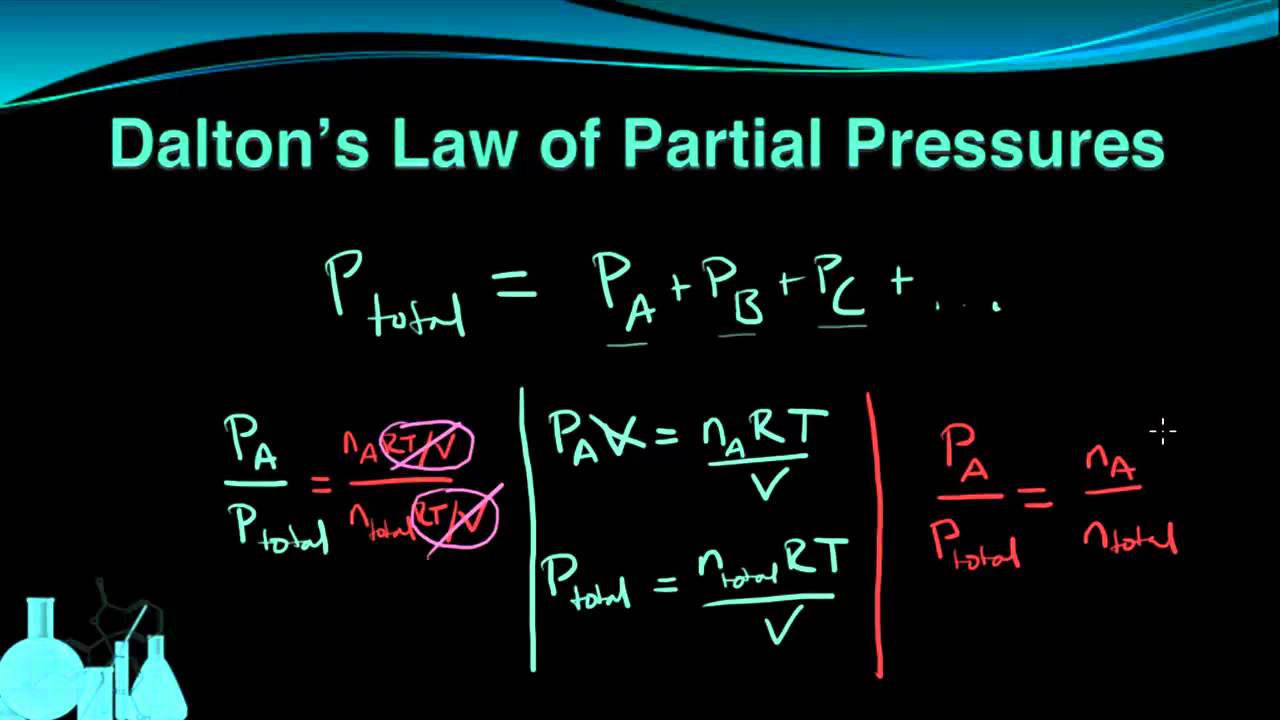
Chemistry 7 6 Dalton S Law Of Partial Pressures Dalton S Law Chemistry Dalton
Formula of Daltons Partial Pressure.

. The pressure exerted by each gas in a mixture is called its partial pressure. Daltons law is also known as the law of partial pressure or Gibbs-Dalton law rarely. The partial pressure is the pressure each gas would exert if it alone occupied the volume of the mixture.
Application of Daltons Law. Daltons Law of partial pressure for moist air can be expressed as. Given by John Dalton.
1 Using symbols I write Daltons Law of. By the symbol P with the symbol of the gas in the subscript. Daltons Law of Partial Pressures states that the total pressure of a gas mixture is the sum of the partial.
Daltons Law of Partial Pressure. What is the total pressure inside the container. This chemistry video tutorial explains the concept of daltons law of partial pressure.
Jump search Pressure attributed component gas mixture mixture gases each constituent gas has partial pressure which the notional pressure that constituent gas alone occupied the. Accordingly why do we say that total pressure is the sum of partial pressures Daltons law of pressure since pressure is intensive just like temperature. It provides the equations plus plenty of examples and practice probl.
According to Daltons law of partial pressures the total pressure exerted by the mixture of gases is the sum of the partial pressure of every existing. P tot p 1 p 2 p 3 p m orp tot n 1 n 2 n 3 n mRTv. Daltons Law of Partial Pressure states that the total pressure exerted by a mixture of gases is equal to the sum of the partial pressures of each individual gases present in the mixture.
Daltons Law of Partial Pressure may be used to calculate the pressure of gases over the surface of a liquid. Both Amagats and Daltons laws predict the properties of gas mixtures. Is it the fact that.
P pa pw 1. The partial pressures of the three gases are 200 atm 300 atm and 400 atm respectively. Where p 1p 2p 3p m Partial pressures of the individual gases in the mixture.
Daltons Law of Partial Pressure. The pressure exerted by each gas is called the partial pressure of that gas. What is Daltons Law of partial pressure and Amagat law.
Daltons Law of Partial Pressure states that the sum of these portions add up to the entire pressure of the container ie the sum of the. The law describes the relationship between the total pressure of a mixture of non. Daltons law of partial pressures assumes that the.
For example P O 2 represents partial pressure of oxygen. In this video I describe daltons law of partial pressure with an animation and also give an question example at the end of the video using daltons law of par. Daltons Law of Partial Pressures is important to diving because the gas mixture that a diver breathes at depth.
Daltons Law of partial pressure.

Dalton S Law Of Partial Pressures Explained

15 12 6 Dalton S Law Of Partial Pressure In Mixtures Of Gases Each Component Gas Behaves Independently Of The Other S In 2022 Ideal Gas Law Molecular Physics Formulas

Dalton S Law Of Partial Pressure Dalton S Law 11th Chemistry Chemistry

Daltons Law Of Partial Pressures Easy Science Dalton S Law Easy Science Organic Chemistry Study
Comments
Post a Comment